02-May-2025
Protein purification and biophysical characterisation is one of the founding and fundamental building blocks of structural biology. As an initial step for understanding the quality of the purified sample before functional and/or structural analysis, or even as the principle sample preparation process for structural studies, these techniques are a crucial asset to the structural biology community. Studies from Guérin et al (2024, and 2025), demonstrates the uses of such techniques.
Borrelia are spirochetes, a form of gram-negative bacteria, responsible for a range of diseases including Lyme disease, or Lyme Borreliosis, the most common tick-borne disease in the world, with an estimation of 700,000 cases per year in the USA and Europe. This study examined two surface proteins expressed in two species of Borrelia, CspZ and FhbA, in species burgdorferi and hermsii respectively.
Borrelia burgdoferi and Borrelia hermsii are both able to escape host immune response in vertebrates thanks to these proteins by interacting with components of the human complement system (which induces an inflammatory response to fight infection by invasive antibodies), namely factor H (FH) and FH-like protein 1 (FHL-1).
This study utilised protein purification and characterisation services available at Robotein in Liège, part of Instruct-BE. They received funded access to the facility through the ISIDORe project; the project aims to deliver access for infectious disease research.
At the Robotein lab, recombinant CspZ and FhbA were expressed in E. coli, with either 15mg (CspZ) or 20mg (FhbA) of protein purified per litre of culture. High-performance liquid chromatography (HPLC) was used to test the purity of these purified proteins, revealing that CspZ was 99% pure, and FhbA 92%. Dynamic light scattering (DLS) was used to test for aggregates and assess the size distribution of the proteins, finding that the CspZ solution was of good quality, free of aggregates, and that the protein rarely formed dimers. However, FhbA much more readily formed multimers – corroborated by size exclusion chromatography–multi-angle light scattering (SEC-MALS) and mass spectrometry. This “could be an intrinsic property of the protein”, but could also be down to the buffer used. In any case, multimerization is likely dynamic based on the protein concentration, and more importantly, FhbA still interacted at high affinities with both FH and FHL-1, even as a multimer.
Circular dichroism (CD) analysis revealed that the purification led to correctly folded secondary structures of the proteins. An NMR stability study on CspZ found that the protein is highly stable at 4 °C for up to 1 month, but can also be stored at −80 °C without impact on its structural integrity. That the protein can be efficiently purified and stored, is crucial for subsequent 3D structural analysis.
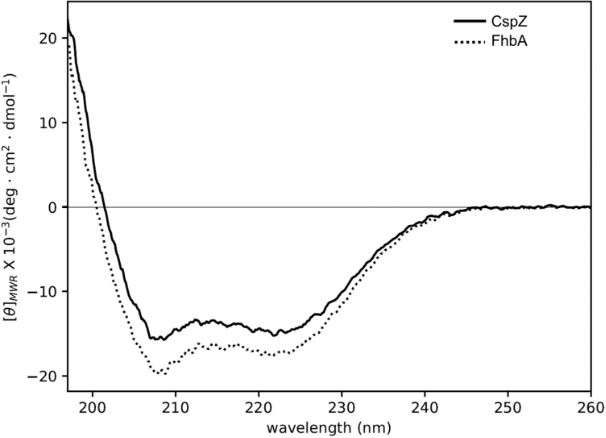
Analysis of the far-UV CD spectrum (Fig. 1) of the two proteins unveiled a well-defined secondary structure organization. Both spectra exhibit two ellipticity minima around 222 and 208 nm, along with a strong positive signal below 200 nm, which are distinct signatures of native proteins with a high content of α-helical structures.
Surface plasmon resonance (SPR) analysis is used to measure the binding affinity of both CspZ and FhbA with the host proteins. It showed that both bacterial proteins bind strongly to FH and FHL-1, with CspZ binding with higher affinity for FHL-1, and the reverse for FhbA. They found also that CspZ does not bind to the 15-20 amino acid region of FH, unlike for FhbA which binds even more strongly in this region.
The 2024 study demonstrates that Borrelia CspZ and FhbA proteins expressed in and purified from E. coli are stable, correctly folded, and, in vitro, bind to human proteins in the complement system. It outlines that two surface proteins clearly bind strongly to the complement system proteins from human host.
The 2025 study aimed to assess the binding to CspZ by DNA aptamers. Aptamers are single-stranded DNA or RNA oligonucleotides that are capable of binding to a wide range of molecules. They are a key diagnostic tool, due to their low toxicity and specific binding mechanism.
The use of cross-over SELEX (Systematic Evolution of Ligands by EXponential enrichment) allowed the team to generate a yield of aptamers that bind to CspZ, before utilising biophysical techniques BioLayer Interferometry (BLI) and SPR to evaluate the affinity of the 13 identified candidate aptamers. Several aptamers formed a very high enrichment, but were found to have a low binding affinity to Borrelia proteins.
These experiments, coupled with dot-blot analysis and followed by CD, found that Aptamers 9 and 10 bind to the soluble recombinant CspZ, forming G4 structures. Furthermore, flow-cytometry and epifluorescence microscopy confirmed that both aptamers interacted with the CspZ protein as it is exposed on the Borrelia surface. This result is significant because these aptamers have the potential to be a detection tool for CspZ, and the process of biophysical studies to identify aptamers that bind to target proteins has broad applications in Lyme borreliosis.
Further structural analysis can now be carried out to uncover the precise binding interface and a potential basis for drug discovery. The study highlights the importance of target protein purification and characterisation studies preliminary to successful selection of aptamers. Through a range of biophysical techniques, it is possible to validate both the structure and the function of the targeted proteins, and thus to envisage the selection of functional new molecular probes.
Read the full papers below: